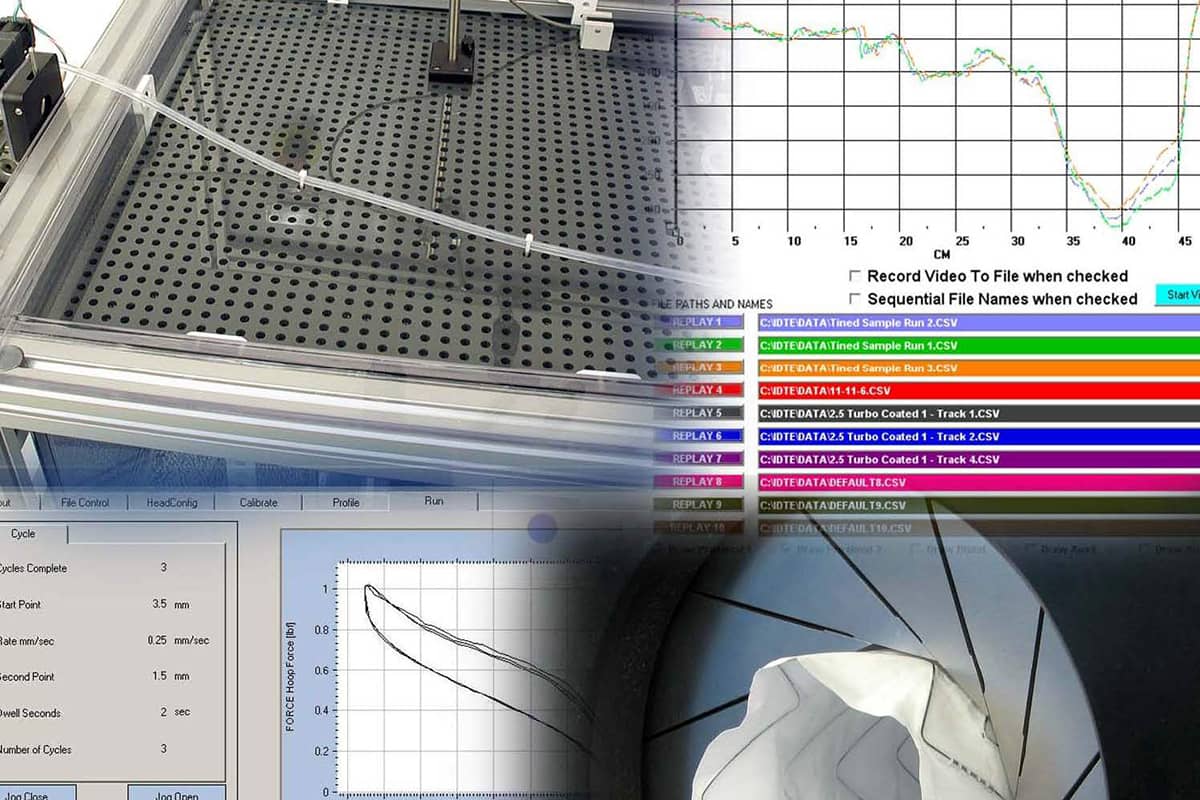
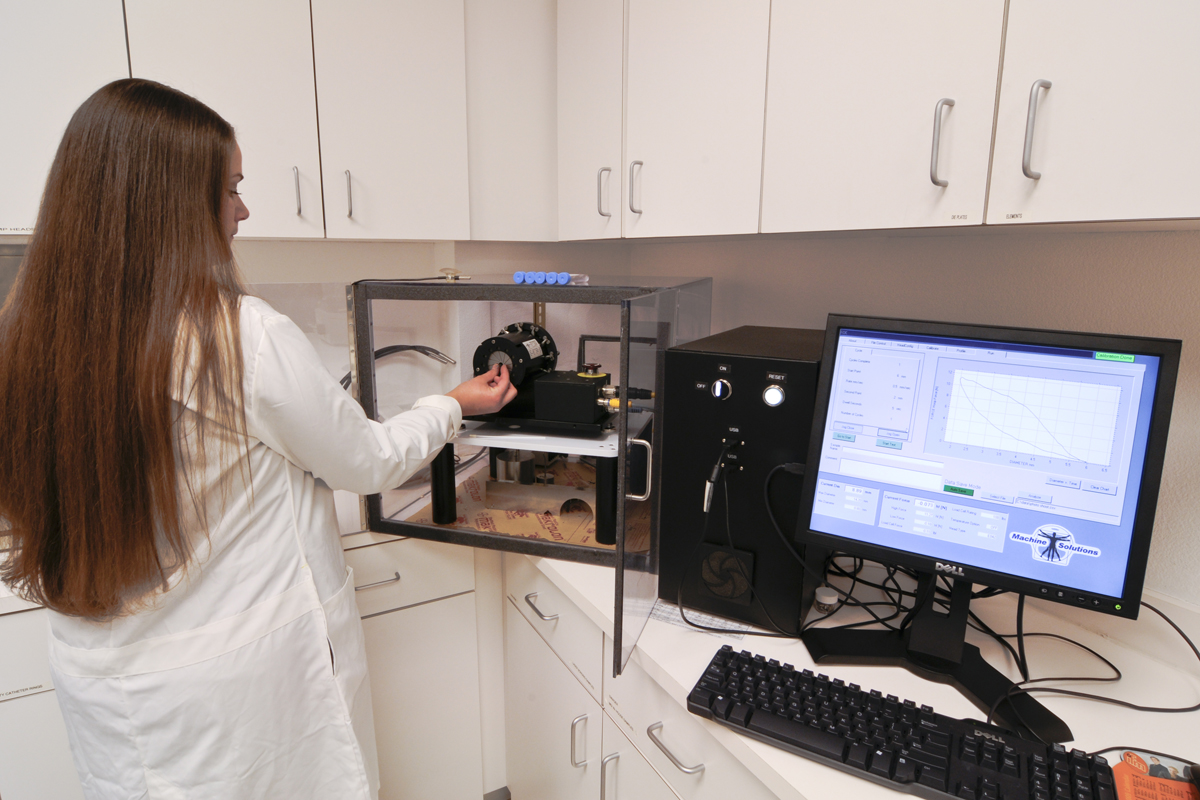
Additional Testing Services
Standard Test Protocols include:
- Balloon burst
- Insertion force measurement
- Balloon fatigue
- Lesion crossability force
- Bifurcation “kissing” stent force
- Push efficiency
- Catheter-guidewire compatibility
- Stent Flexibility
- Catheter component tensile
- System burst strength
- Tip pull
- Catheter profile
- Torque strength
- Compliance
- Torqueability
- Crossing profile
- Track force
- Flex (kink)
- Vessel straightening forces
- Individual bond strength
- Inflation/deflation time
Spec. Sheet Download
© 2025 MSI. All rights reserved. Designed and developed by: Tension Design