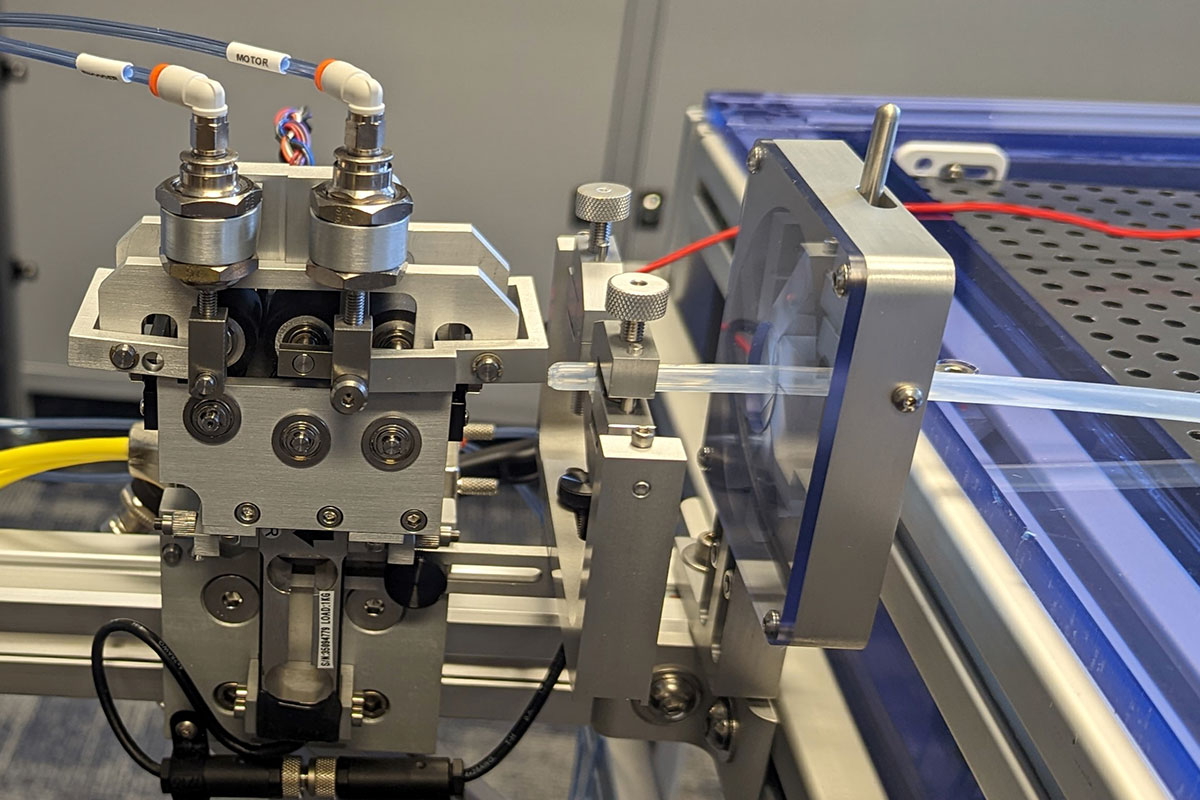
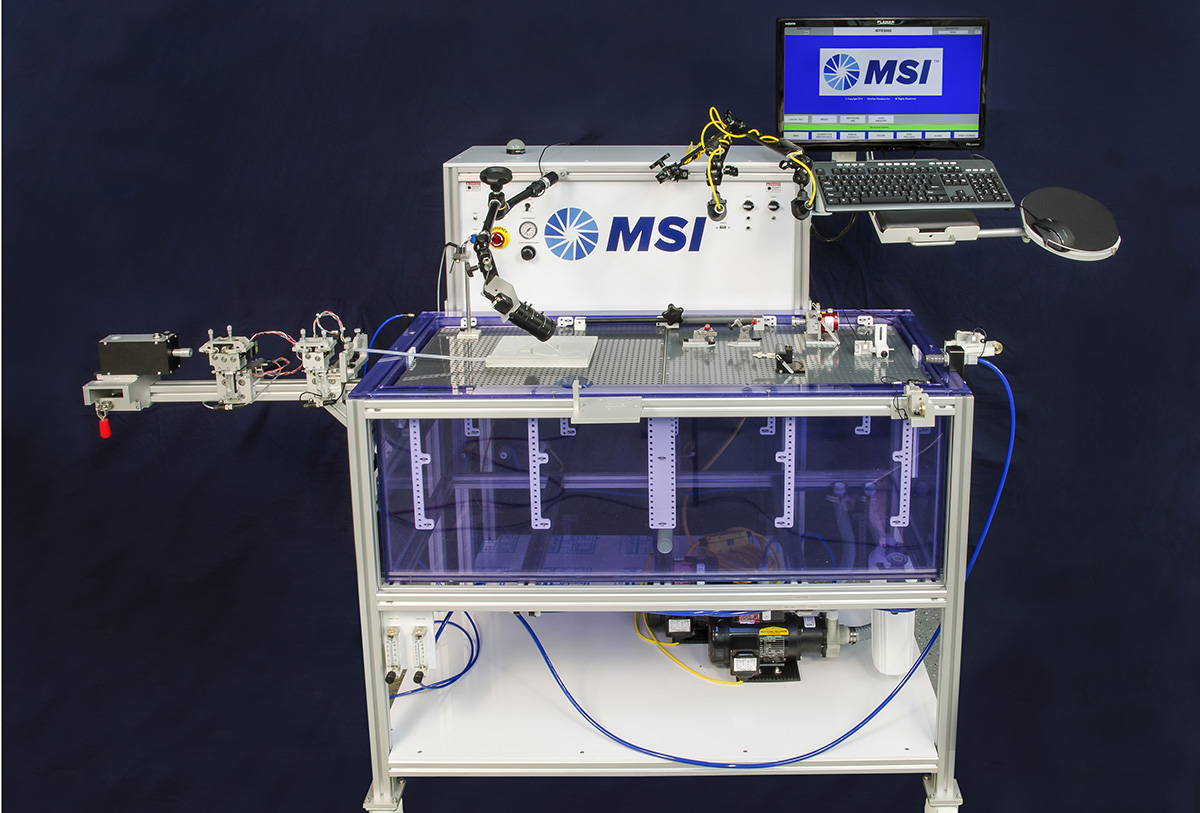
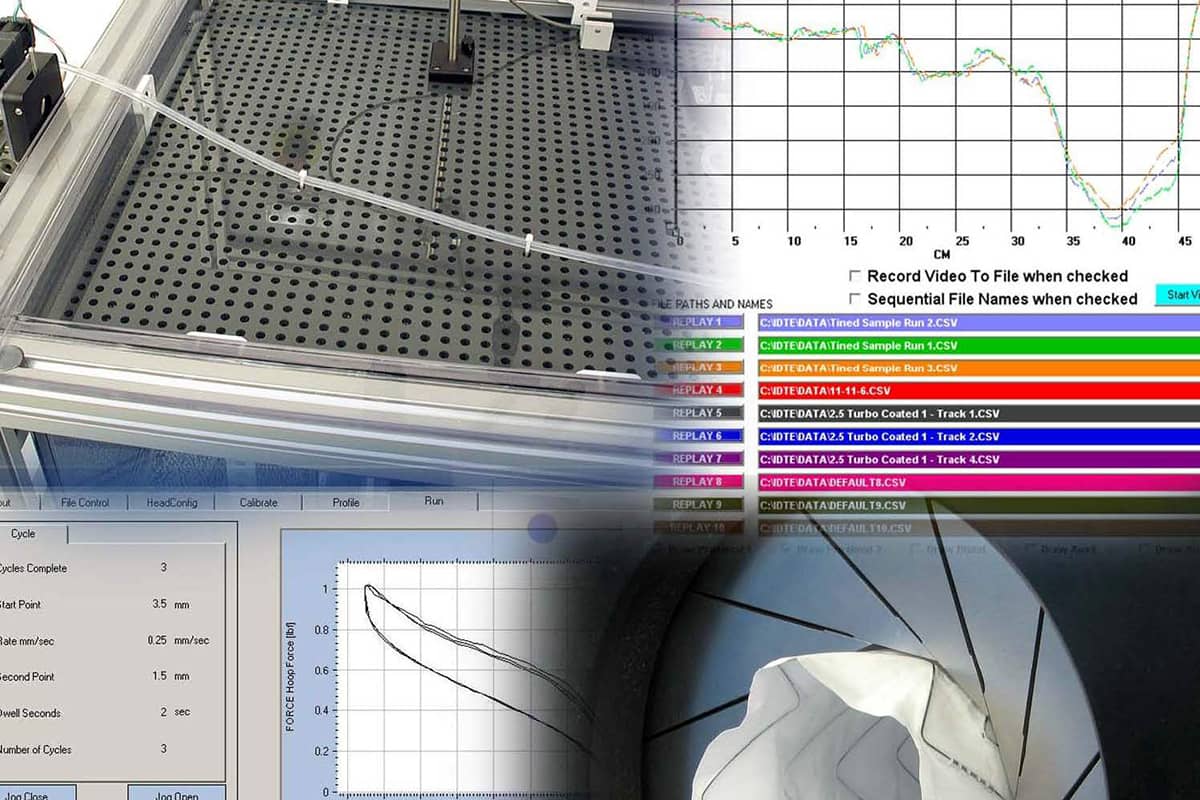
Trackability Test Equipment
Standard test methods include:
- Track force
- Lesion crossability force
- Catheter-guide wire compatability
- Flexibility/kink force
- Bifurcation “kissing” stent force
- Insertion force measurement
- Push efficiency (requires optional distal load cell)
© 2025 MSI. All rights reserved. Designed and developed by: Tension Design