
ISO 10555 Catheter Testing
What is Catheter Testing?
Catheters or balloon catheters are devices used during minimally invasive interventional procedures to navigate the vasculature to target clinical sites for diagnosis or treatment procedures.
Intravascular catheters have become essential to the practice of modern medicine by improving treatment methods, patient care, and quality of life.
While catheter designs evolve and improve so to must the testing of these devices. Balloon catheters require rigorous functional and failure testing to ensure efficacy, safety, and performance.
Catheter & Balloon Test Methods
There are a variety of methods used to evaluate the properties of catheters and balloons.
ISO 10555 outlines general requirements for testing intravascular sterile use catheters.
We offer comprehensive testing services for catheters according to
FDA guidance document recommendations and ISO standards.
Device Testing and Analytical LabTest MethodsISO and ASTM Testing Standards
For 3 decades our carefully engineered Testing solutions have been relied upon to test medical devices the world over.
Test your medical devices in accordance with ISO and ASTM using our wide range of available solutions.
Organized for your convenience by testing standards and the correlating testing solution is a cross reference table to ensure your medical device testing compliance.
ISO 10555 – 1 Intravascular Catheters – Sterile and single use – General Requirements
- Annex B – Method for Determining Peak Tensile Force – Schedule MSI Lab Testing Services.
- Annex C – Test method for liquid leakage under pressure – CRESCENT HYDRAULIC BURST TESTER
- Annex D – Test method for air leakage into hub assembly during aspiration – Schedule MSI Lab Testing Services
- Annex F – Test for burst pressure under static conditions – CRESCENT HYDRAULIC BURST TESTER
ISO 10555 – 2 Intravascular Catheters – Sterile and single use – Angiographic Catheters – Crescent Hydraulic Burst Tester.
ISO 10555 – 3 Intravascular Catheters – Sterile and single use – Central Venous Catheters – Crescent Hydraulic Burst Tester.
ISO 10555 – 4 Intravascular Catheters – Sterile and single use – Balloon Dilation Catheters – Crescent Hydraulic Burst Tester.
- Annex A – Test method for balloon rated burst pressure (RBP) – CRESCENT HYDRAULIC BURST TESTER
- Annex B – Balloon fatigue test for freedom from leakage and damage on inflation – CRESCENT HYDRAULIC BURST TESTER
- Annex C – Deflation time – CRESCENT HYDRAULIC BURST TESTER
- Annex D – Test method for balloon diameter to inflation pressure ISO – CRESCENT HYDRAULIC BURST TESTER
- Annex E – Balloon material selection guidelines – longitudinal burst with no fragmentation – hydraulic testing preferred
ISO 10555 – 5 Intravascular Catheters – Sterile and single use – Over-needle peripheral catheters
- Annex D – Determination of liquid leakage from vent fitting – CRESCENT HYDRAULIC BURST TESTER
ISO 11070 – Sterile single use intravascular introducers, dilators, and guidewires
- Annex C – Method for determining peak tensile force of introducer catheters, sheath introducers, and dilators – Schedule MSI lab testing services
- Annex D – Test method for liquid leakage from sheath introducers under pressure – CRESCENT HYDRAULIC BURST TESTER
- Annex E – Test method for liquid leakage through haemostasis valves of sheath introducers – CRESCENT HYDRAULIC BURST TESTER
ISO 80369 – 3 Small-bore connectors for liquids and gases: Connectors for Enteral applications
- 6.1.2 Fluid leakage by pressure decay – CRESCENT HYDRAULIC BURST TESTER
- 6.1.3 Positive Pressure liquid leakage – CRESCENT HYDRAULIC BURST TESTER
ISO 80369 – 3 Small-bore connectors for liquids and gases: Connectors for Enteral applications
- 6.1.2 Fluid leakage by pressure decay – CRESCENT HYDRAULIC BURST TESTER
- 6.1.3 Positive Pressure liquid leakage – CRESCENT HYDRAULIC BURST TESTER
ISO 594 – 1 and 2 – Conical Fittings with a 6%(Luer) taper for syringes, needles, and certain other medical equipment
- 594-1 – 5.2 – Test method for liquid leakage from the conical fitting assembly under pressure – CRESCENT HYDRAULIC BURST TESTER
- 594-2 – 5.2 – Test method for liquid leakage from fitting assembly under pressure – CRESCENT HYDRAULIC BURST TESTER
ISO 7198 – Cardiovascular implants and extracorporeal systems – Vascular prostheses , tubular vascular grafts and vascular patches
- A.5.1.4 – Water entry pressure – CRESCENT HYDRAULIC BURST TESTER
- A.5.2.2 – Pressurized burst strength – CRESCENT HYDRAULIC BURST TESTER
ISO 25539-1 – Cardiovascular implants – Endovascular prostheses
- D.5.1.1.1 – Balloon Burst Pressure for non-compliant balloons – CRESCENT HYDRAULIC BURST TESTER
- D.5.1.1.2 – Balloon deflation times – CRESCENT HYDRAULIC BURST TESTER
- D.5.1.1.3 – Balloon rated fatigue – Crescent Design Hydraulic Burst Tester
- D.5.1.3 – Dislodgement force – MSI Stent Retention Tester
- D.5.1.4 – Force to deploy – MSI Radial Force Tester
- D.5.1.5 – Simulated Use – MSI Simulated Use Tester
- D.5.1.6 – Tensile bond strength – Schedule MSI lab testing services
- D.5.1.7 – Torsional bond strength – MSI Simulated Use Tester
- D.5.2.5.3 – Crush resistance with radially applied load – MSI Radial Force Tester
- D.5.2.5.4 – Radial force – MSI Radial Force Tester
- D.5.2.5.5 – Resistance to kinking (flexibility) – MSI Simulated Use Tester
ISO 25539-2 – Vascular Stents
- D.5.2.2.3 – Balloon rated burst pressure – CRESCENT HYDRAULIC BURST TESTER
- D.5.2.2.4 – Balloon rated fatigue – CRESCENT HYDRAULIC BURST TESTER
- D.5.2.3 – Dimensional verification of the stent system (ASTMF2081) – CRESCENT HYDRAULIC BURST TESTER
- D.5.2.4 – Dislodgement force – MSI Stent Retention Tester (ASTM F2394)
- D.5.2.5 – Force to deploy (self expanding stents) – MSI Radial Force Tester
- D.5.2.5.3 – Crush resistance with radially applied load – MSI Radial Force Tester
- D.5.2.5.4 – Radial force – MSI Radial Force Tester
- D.5.2.5.5 – Resistance to kinking (flexibility) – MSI Simulated Use Tester
- D.5.2.8 – Simulated use – MSI Simulated Use Tester
- D.5.2.9 – Tensile bond strength – Schedule MSI lab testing services
- D.5.2.10 – Torsional bond strength – MSI Simulated Use Tester
- D.5.3.4.3 – Crush resistance with radially applied load – MSI Radial Force Tester (ASTM F3067)
- D.5.3.4.4 – Radial force (self-expanding stents) – MSI Radial Force Tester (ASTM F3067)
- D.5.3.4.5 – Kink resistance (flexibility) – MSI Simulated Use Tester
- D.5.3.5.3 – Stent length – Schedule MSI Lab Testing Services
- D.5.3.5.4 – Recoil (balloon expandable stents) – Schedule MSI Lab Testing Services
ASTM F2394-07 Preconditioning of stent on the deployment system prior to retention testing – MSI Simulated Use Tester and MSI Stent Retention Tester
ASTM F623 Standard performance specification for Foley Catheter – MSI Simulated Use Tester
ASTM F2081 Standard Guide for Characterization and Presentation of the Dimensional Attributes of Vascular Stents – CRESCENT HYDRAULIC BURST TESTER
ASTM F2394 Standard Guide for Measuring securement of Balloon Expandable Vascular Stent mounted on delivery system – MSI Simulated Use Tester
ASTM F3067 Guide for Radial Loading of Balloon Expandable and Self Expanding Vascular Stents – MSI Radial Force Tester
ASTM F2606-08 Standard Guide for Three-Point Bending of Balloon Expandable Vascular Stents and Stent Systems – Schedule MSI lab testing services.
Non- Clinical Engineering Tests and Recommended Labeling of Intravascular Stents and Associated Delivery Systems – Schedule MSI Lab Testing services.
Class II Special Controls Guidance Document: Vascular and Neurovascular Embolization Devices – Schedule MSI Lab Testing Services
Class II Special Controls Guidance Document for Certain Percutaneous Transluminal Coronary Angioplasty (PTCA) Catheters – Schedule MSI Lab Testing Services
Title 21 -Food and Drugs, Chapter 1 – Food and Drug Administration, Department of Health and Human Services, Subchapter A-General, Part 58 – Good Laboratory Practice for Non-clinical Laboratory Studies – Schedule MSI Lab Testing Services
Listed here are a variety of standard test methods our experienced team offers to evaluate the performance of Catheters, Balloon catheters, or accessory devices (guidewires, guide catheters, endoscopes, stents…). These test methods were designed following the outlined general requirements in the ISO 10555 and ISO 11070 standards, as well as the FDA 824 guidance document. MSI Lab follows best professional practices and requires customer approval of study plans and protocols prior to performing testing.
- Balloon Burst, Leak, Compliance (HBLT) Testing
- Radial Expansion Testing
- Stent Retention Tester
- Motorized Push/ Pull Tester
- Dimensional Analysis/ Verification
- Torque Characteristics
- Simulated Use Testing
Radial Expansion Testing
Utilizing MSI’s Radial expansion testing equipment, the RX650, following stepped parameters that control and record the Iris diameter and speed MSI Labs quantitatively collect the radial resistive forces and the Chronic outward forces that a stent experiences during a run process.
Standard test methods include:
- Radial compression/expansion cycle testing
- Stent deployment forces
Related Standards and Guides:
- ISO 25539
- ISO/TS15539
- FDA guidance 1545
Stent Retention Tester
Utilizing MSI’s Stent Retention equipment, the SR1000, following stepped parameters that control and record retention diameter, distance, and speed MSI Labs quantitatively collect forces required dislodge a balloon expandable stent from the balloon catheter.
Standard test methods include:
- Stent Securement
Related Standards and Guides:
- ISO Standard 25539
- ASTM F2394
Motorized Push/ Pull Tester
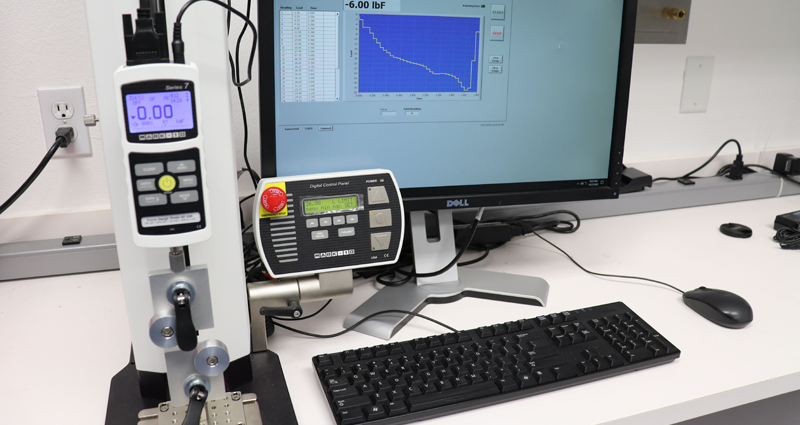
Utilizing our in-house Motorized push/ pull tester with data collection, MSI labs can control to move speed and distance while collecting force distance and speed data. This method of testing is for all ASTM and ISO linear tension and compression testing.
- 3 point bend
- Tension/ Compression
- Tip stiffness
- Packaging
Related Standards and Guides:
- ISO Standard 25539
- ASTM F2081
- ASTM F2606
Dimensional Analysis/ Verification
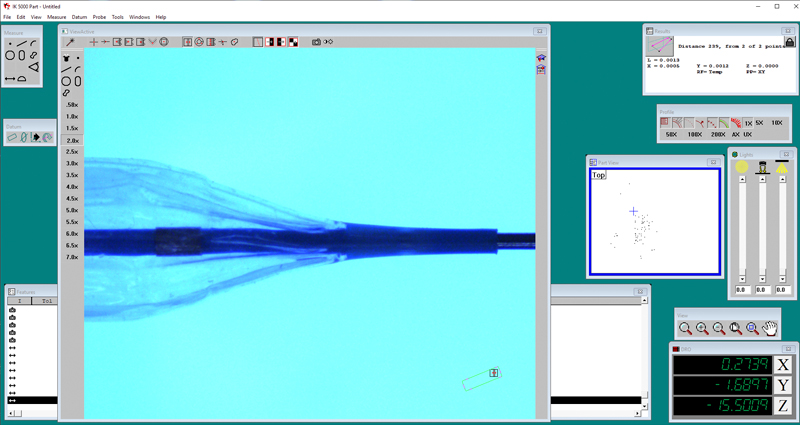
Utilizing calibrated measurement equipment, MSI Labs will analysis and record product measurements at specified locations as a standalone dimensional verification or pre and/or post simulated use or other testing method.
Related Standards and Guides:
- ISO Standard 25539
- ASTM F2081
Torque Characteristics
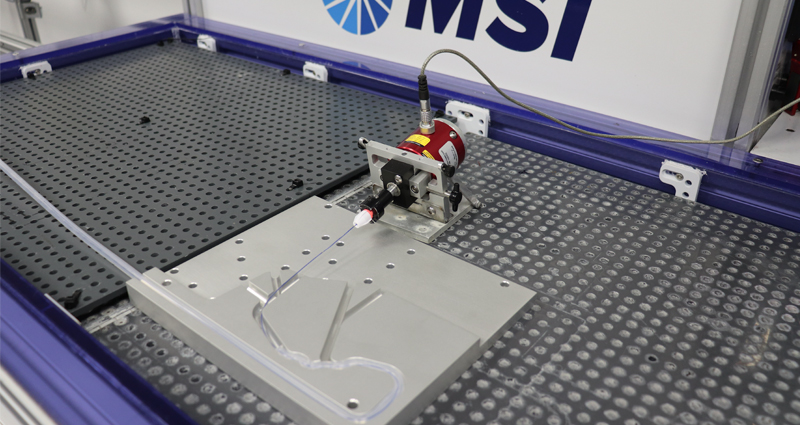
Utilizing MSI’s simulated use testing equipment, the IDTE, following stepped parameters that control and record proximal rotational direction and speed MSI Labs quantitatively collect proximal forces and rotations while also recording distal forces or rotations.
Standard test methods include:
- Torque strength
- Rotational response
- Torque to failure
- Rotational Bond strength
Related Standards and Guides:
- ISO 10555
- ISO 25539
- ISO/TS15539
- ASTM F2394
Simulated Use Testing
Utilizing MSI’s simulated use testing equipment, the IDTE, following stepped parameters that control and record insertion/withdraw- distance, speed, and delay MSI Labs quantitatively collect forces required to track a catheter or device through a tortuous path that mimics challenging in vivo physiologic/anatomic conditions.
Standard test methods include:
- Simulated use
- Lesion cross-ability force
- Push efficiency
- Catheter-guide wire compatibility
- Flexibility/kink force
- Bifurcation “kissing” stent force
- Insertion force measurement
- Preconditioning cycles
Related Standards and Guides:
- ISO 10555
- ISO 25539
- ISO/TS15539
- ASTM F2394
Device Testing and Analytical Lab
Test Before You Invest.
Development of robust, rugged testing and analytical procedures ensure the quality and safety of your medical devices. Rely on our experienced testing team who takes care to follow ISO standards and those of regulatory agencies worldwide. We partner with you virtually or provide a hands-on experience to develop a test method and compliant testing of balloon catheters. Reasonably priced for both R&D customers and full manufactures, this Non-Certified lab provides the quickest turn-time available.
Your satisfaction and confidence in our products are our top priorities, and we are excited to provide you with this opportunity to experience the quality and efficiency of our offerings firsthand. Get In Touch Today
-
Send In Samples or Schedule A Lab Day
-
Test Before You Invest
-
Real-Time Data Reporting














 HYDRAULIC BURST AND LEAK TESTING EQUIPMENT
HYDRAULIC BURST AND LEAK TESTING EQUIPMENT  Stent Securement Testing
Stent Securement Testing  Radial Force & Hoop Force Testing
Radial Force & Hoop Force Testing 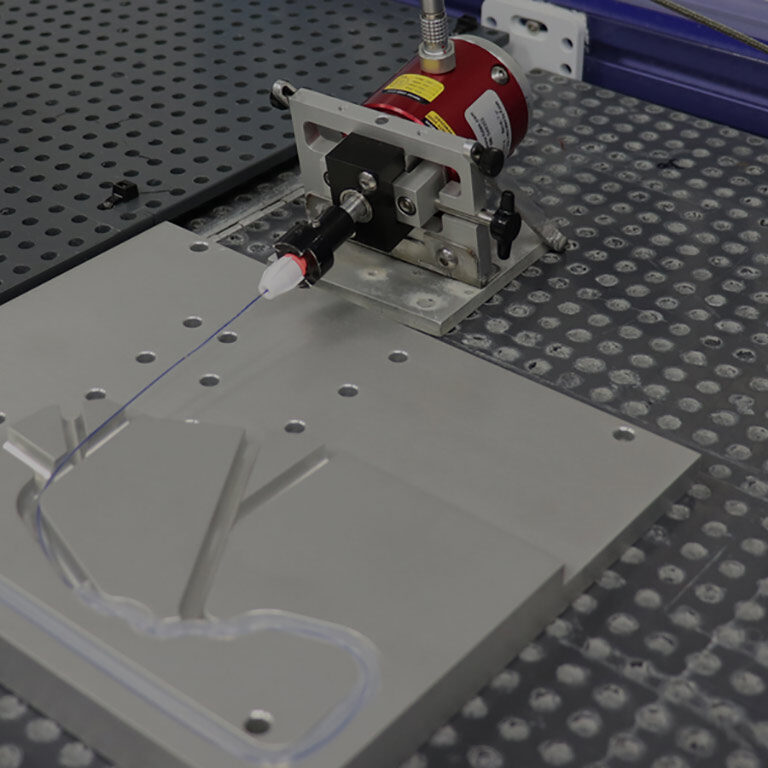 Catheter Testing Equipment
Catheter Testing Equipment