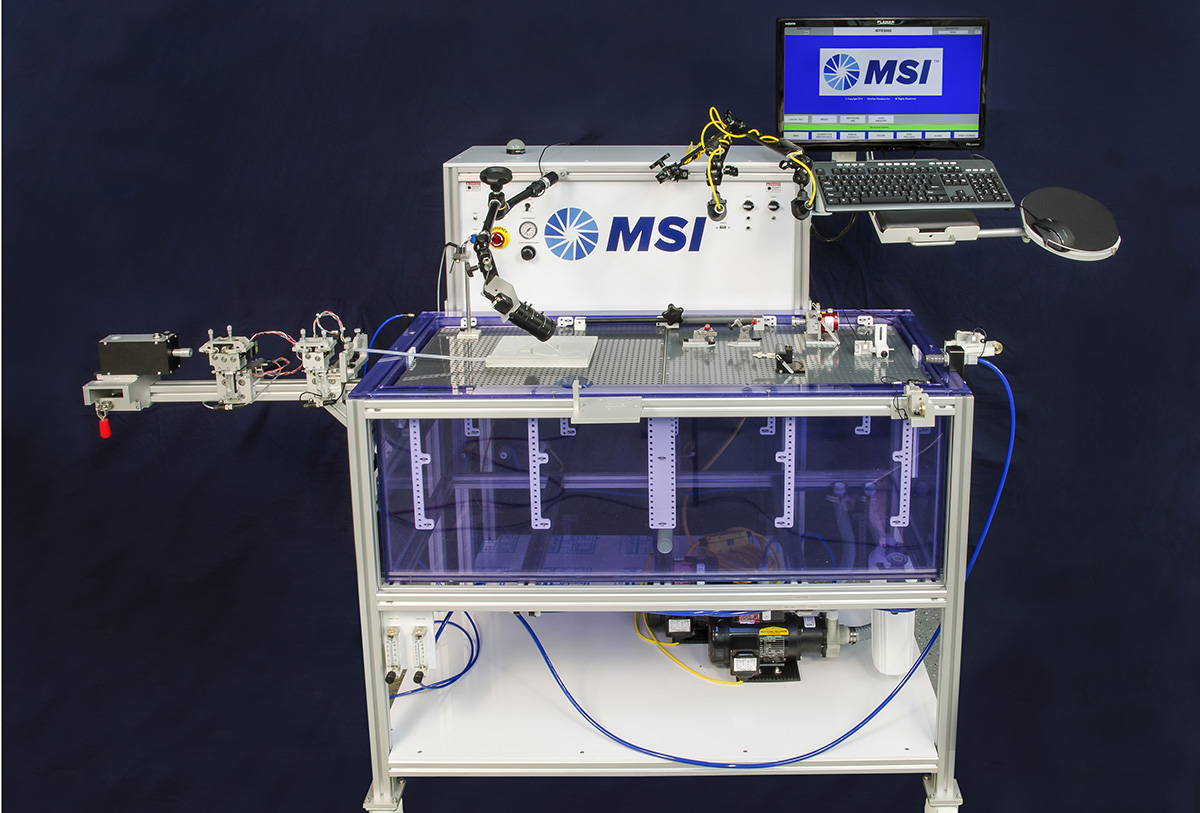
Device Testing Equipment
Standard test methods include:
- Track force
- Lesion crossability force
- Push efficiency
- Catheter-guide wire compatability
- Flexibility/kink force
- Bifurcation “kissing” stent force
- Torque strength
- Rotational response
- Torque to failure
- Tip stiffness
- Insertion force measurement
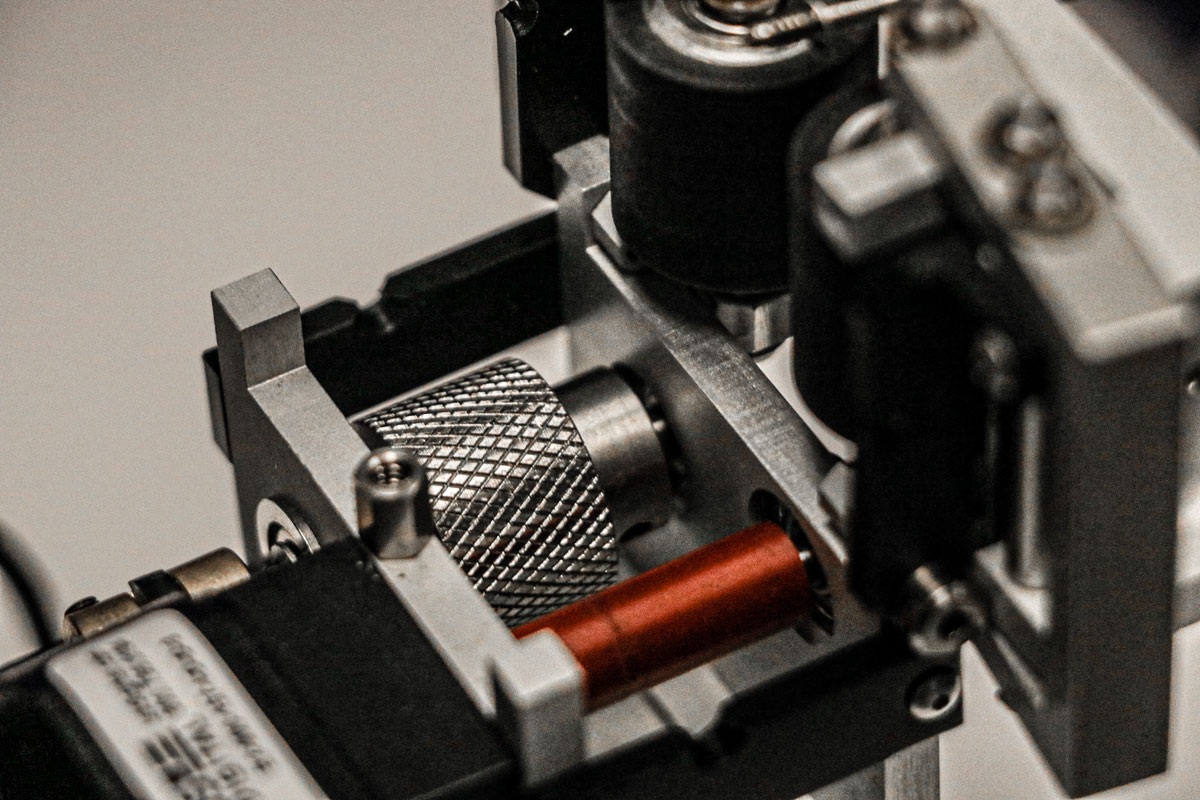
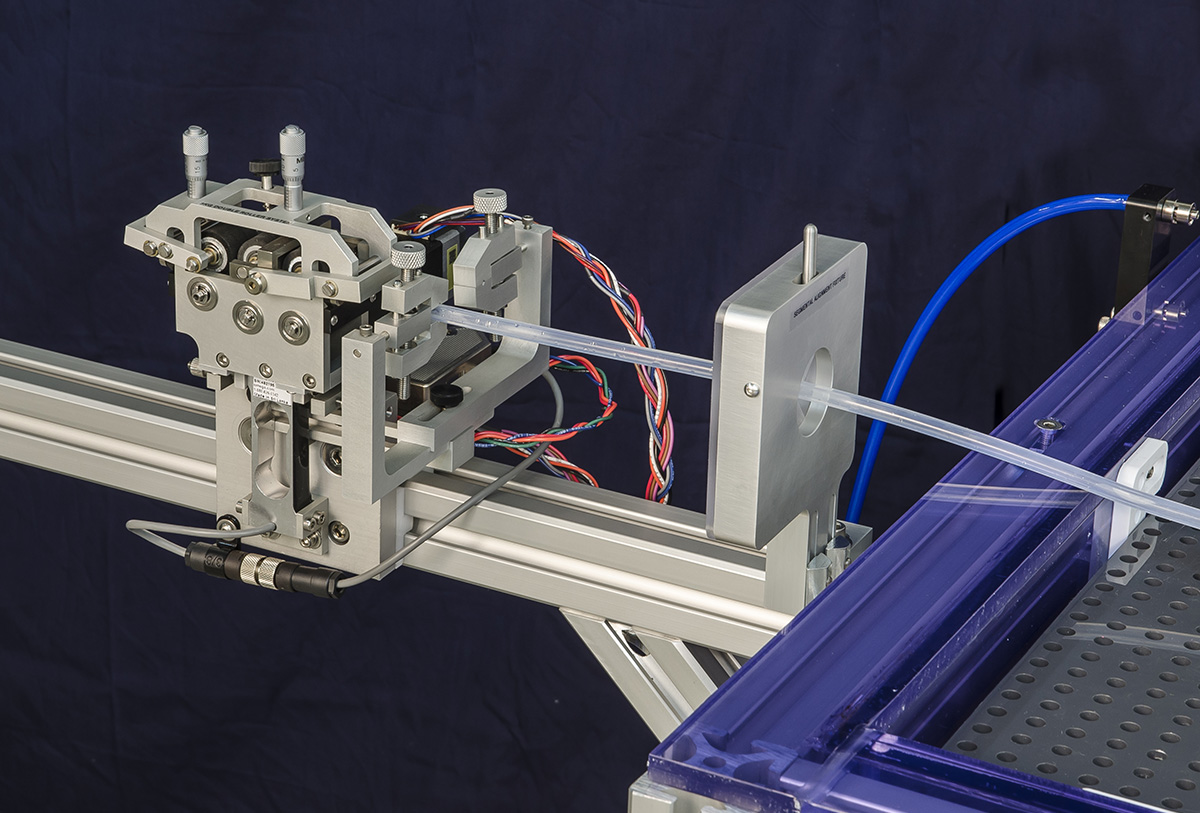
Available Upgrades
- Upgrade the drive rollers on your IDTE Proximal roller system to the stainless steel and eliminate product “slipping” for your coated catheter testing.
- The knurled stainless steel drive rollers ensure the drive rollers keep a strong grip on your product even when wet.
Spec. Sheet Download
© 2025 MSI. All rights reserved. Designed and developed by: Tension Design