Radial Force Testing (wet)
Description
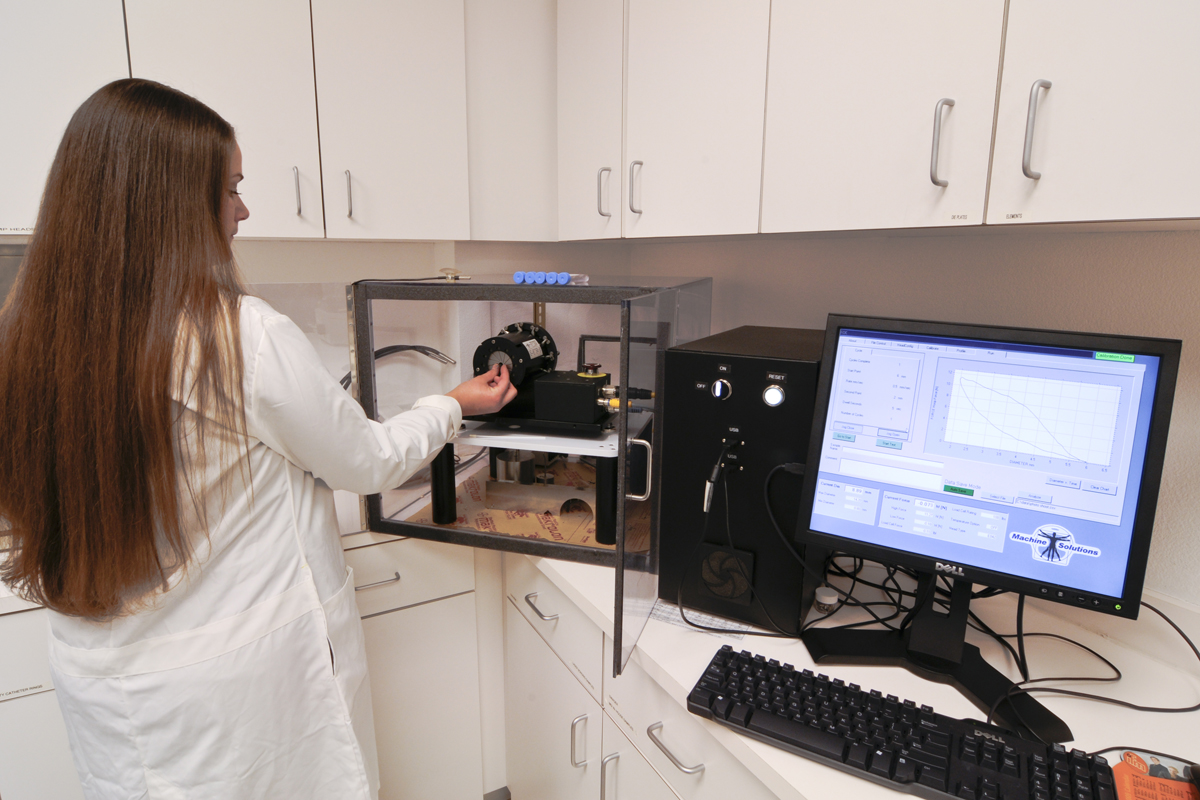
The RX750/850 is designed to measure and record radial stiffness and strength of self-expanding and balloon expandable devices within a temperature-controlled water bath. Many new devices are comprised of materials with variable mechanical properties that are temperature-dependent. MSI’s off-the-shelf testing solution measures force decay over time and radial strength and stiffness in an aqueous environment. The RX750/850 uses the same integrated high-speed data acquisition system and proprietary software as the RX550/650, generating repeatable, reproducible data to facilitate knowledgeable and rapid device design changes.
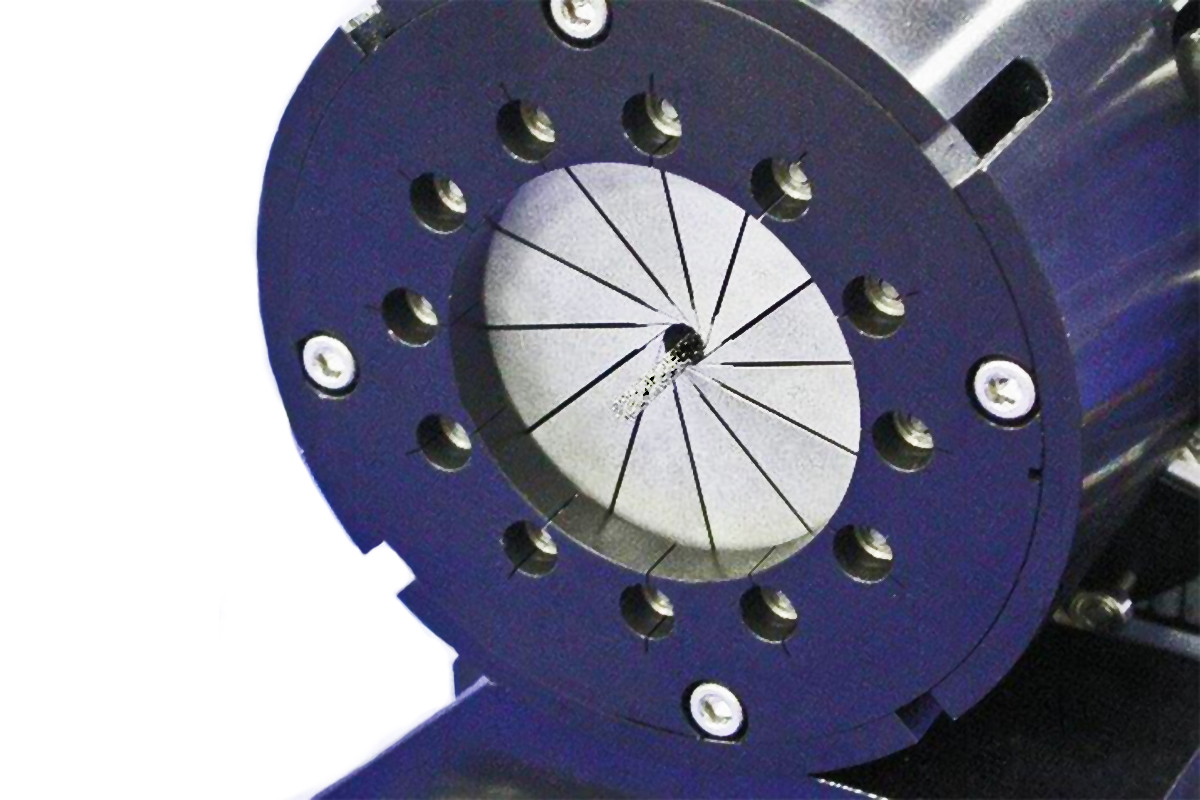
MSI’s proprietary segmental compression mechanism is the core technology in the RX equipment, which collects data from 12 product contact points providing uniform radial measurement. The RX750/850 system has an optional dual tank base that simulates the temperature changes that affect products during loading and body deployment. The RX equipment uses an encoder for diameter accuracy, a linear actuator for activation, and a precision roller bearing system that provides a low friction testing environment. Test results can be used for regulatory submissions, competitive product testing, R & D device evaluation, and inline manufacturing quality assurance.
MSI’s RX equipment supports conformity with the following international recommendations:
- S. Food and Drug Administration. (2010.) Non-Clinical Tests and Recommended Labeling for Intravascular Stents and Associated Delivery Systems. Center for Devices and Radiological Health, Silver Spring, MD, USA.
- ISO (2003). ISO 25539-1 Cardiovascular implants – Endovascular devices – Part 1: Endovascular prostheses. International Organization for Standardization, Geneva, Switzerland.
- ISO (2008). ISO 25539-2 Cardiovascular implants – Endovascular devices – Part 2: Vascular Stents. International Organization for Standardization, Geneva, Switzerland.
- ISO (2000). ISO/TS 15539 Cardiovascular implants – Endovascular prostheses. International Organization for Standardization, Geneva, Switzerland.
Product Features
- Submersible segmental compression mechanism
- Ideal for testing polymer scaffolds and living tissue heart valves
- Polymer scaffold will have different radial strength when tested in aqueous environment
- Repeatable and reproducible data
- Software capable of replaying multiple data sets for fast and easy comparisons
- Force control capability for measuring diameter creep over time
- Data output optimized for spreadsheet analysis
- Interchangeable load cells for optimal force resolution