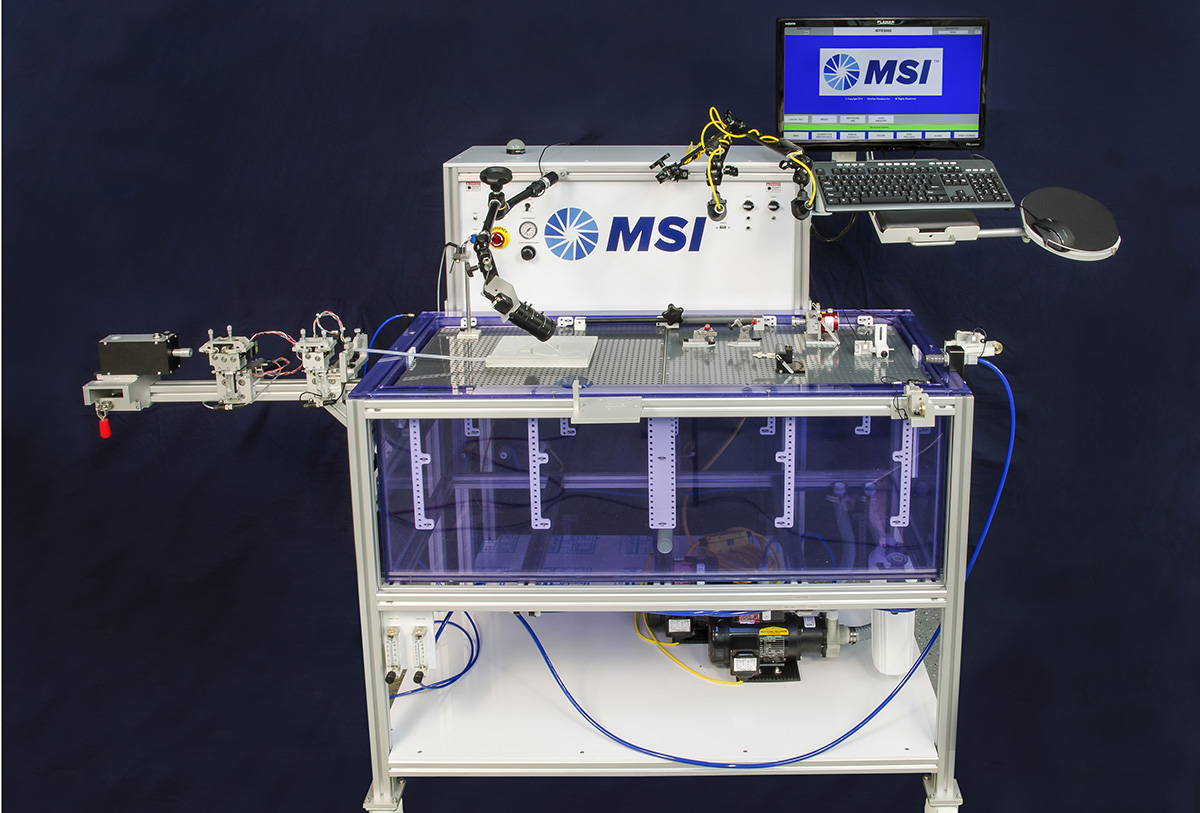
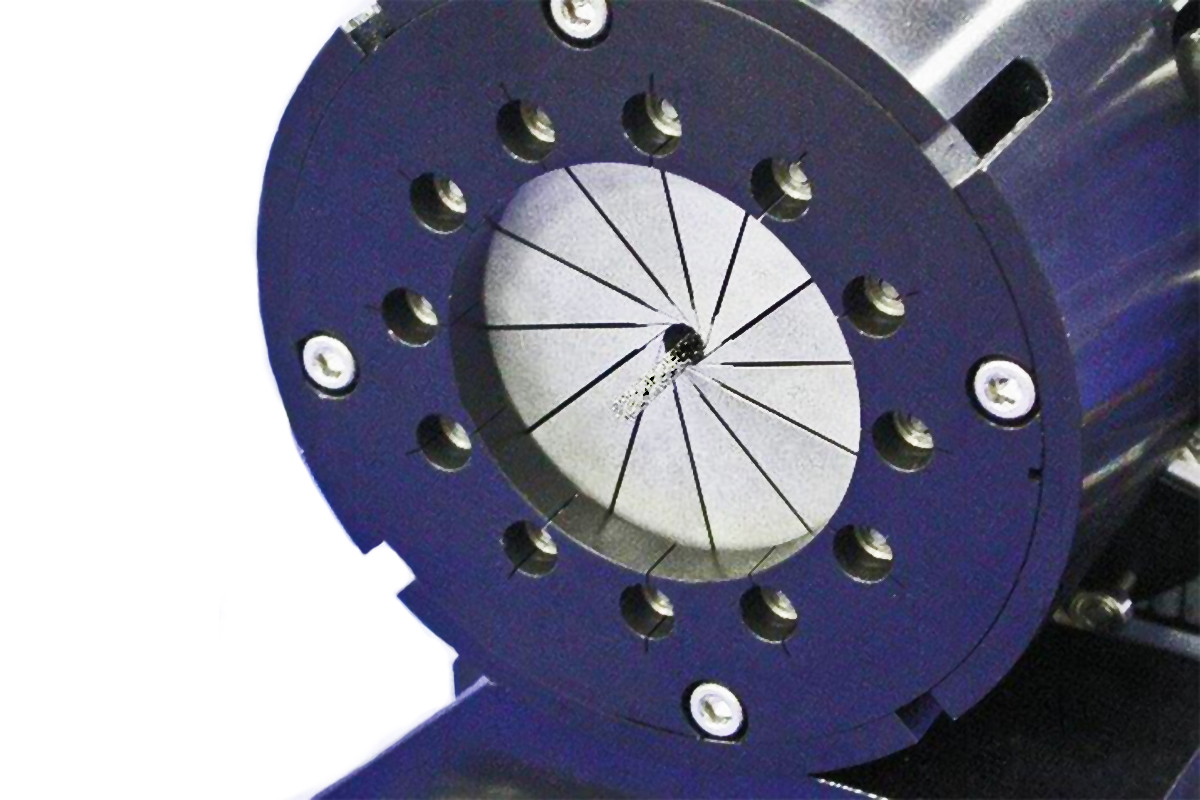
Stent Securement Testing
Product Features
- Proven to have lowest standard deviation in test results compared to tape and shim methods
- Reduce the number of samples required to get statistically significant results
- Allows R&D team to optimize stent crimping process settings by providing meaningful test data
- Designed to meet requirements of ASTM F2394-07 (2013) test standard
- Automatic self-centering design allows any operator to get repeatable results
- Adjustable shim diameter to minimize frictional impacts of catheter
- Video assist with sample alignment and video capture of test
Spec. Sheet Download
© 2026 MSI. All rights reserved. Designed and developed by: Tension Design